
Joining the lab
We are always looking for enthusiastic people who enjoy the science and want to work on important questions. Don’t be concerned if you are coming from a different background or subject – this will allow you to tackle our questions from a new perspective – all you have to bring is genuine curiosity, a proactive approach and team spirit.
Postdoctoral fellows
Talented third-year PhD students and junior postdocs are welcome to be in touch for prospective fellowship applications. We are particularly excited to receive applications for the following projects:
Bioprinting human embryo models to delineate gastrulation and organogenesis
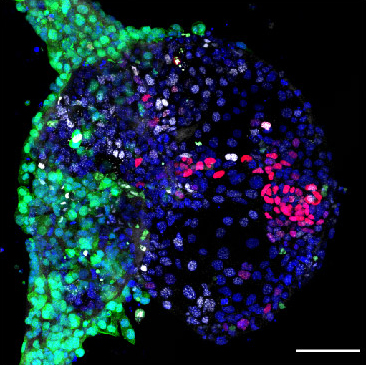 The Boroviak lab is recruiting highly enthusiastic individuals to carry out ground-breaking research on bioprinting human embryo models.
The Boroviak lab is recruiting highly enthusiastic individuals to carry out ground-breaking research on bioprinting human embryo models.
We will tackle the origin of human body axis formation, which occurs during the second week of gestation. Deeply embedded within extraembryonic tissues, gastrulation transforms the embryonic disc into three germ layers and organizes the future body plan. All of these events are essential for healthy embryo development, but in human they have been notoriously hard to study for ethical and technical reasons.
Building on our insights from spatial profiling of primate gastrulation in utero (Bergmann et al., Nature 2022), the postholder will pioneer next-generation human embryo models to functionally dissect germ layer formation. The research associate will be responsible for driving various bioprinting approaches to emulate embryogenesis in vitro. Validation includes single-cell transcriptomics and spatial-identity-mapping to our existing embryo datasets. Ultimately, the goal is to interrogate the molecular cross-talk between embryonic and extraembryonic lineages with human and marmoset reporter and knock-out pluripotent stem cell lines in bioprinted embryo models.
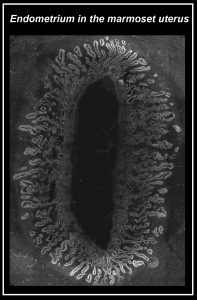 The artificial womb – towards an authentic uterine environment capable of supporting non-human primate embryo implantation
The artificial womb – towards an authentic uterine environment capable of supporting non-human primate embryo implantation
Recent breakthroughs in organoid culture have allowed stable propagation of hormone-responsive human endometrium (Turco et al., 2017, Nature Cell Biology). However, it remains unclear whether endometrial cultures are able to support embryo implantation in a dish. Here, we aim to build on the advances in the organoid system and derive non-human primate endometrial and stromal cell lines from the marmoset uterus. Our goal is to combine endometrial and stromal cultures to reconstruct the uterine environment for natural and synthetic marmoset embryo implantation ex vivo. This will provide a powerful platform to functionally investigate primate implantation and the earliest steps of placental development.
Funding possibilities include EMBO Long-term Fellowships, HFSP, Marie Curie or Royal Society Newton International Fellowships. Postdocs (up to five years within the award of their PhD) aiming to establish their independent research within the next two years are eligible to apply for a Next Generation Fellowship at the Centre for Trophoblast Research.
After an initial interview, the successful candidate will prepare his/her application with help and guidance of the lab head. Fellowships are competitive and require patience, so the sooner you get in touch the better. Organisation and development of a solid research proposal well ahead of the submission deadlines are key to success.
Applications should include a cover letter, briefly outlining why you want to work with us, your CV as well as contact details of two referees and can be sent directly to Thorsten Edwin Boroviak (teb45@cam.ac.uk).
PhD students
Our laboratory participates in various PhD programmes, including the WT-MRC Cambridge Stem Cell Institute, MRC or BBSRC funded programmes.
If you are interested, get in touch and we can apply directly to one of these programmes. Note that if you would like to start your PhD in October, you will need to contact us in the autumn of the previous year.
Master / Bachelor / Summer students
For outstanding candidates there is a selection of smaller projects available.
- A screen for primate-specific regulators of naïve pluripotency
Mammalian preimplantation development establishes the founding population of the entire foetus in the pluripotent epiblast at the late blastocyst stage. This is a highly adaptive process, subject to distinct selective pressures. While the mouse model has been instrumental for our understanding of mammalian development, comparatively little is known about early human and non-human primate embryogenesis.
Recently, we generated a comprehensive single-cell RNA-seq dataset for cross-species analysis of human, marmoset and mouse preimplantation development. Compiling stage-matched transcriptomes allowed us to delineate a candidate set of primate-specific genes expressed in the pluripotent compartment of the embryo. In this project, we will carry out a high-throughput siRNA-screen in marmoset conventional and naïve pluripotent cells to functionally assess primate-specific pluripotency.
- Investigating the mechanosensitivity of naïve primate embryonic stem cells
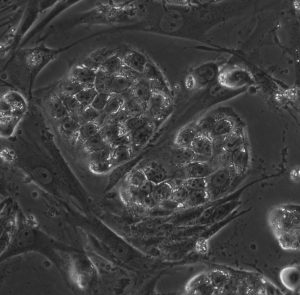 The transition from the naïve to primed pluripotent state in the mammalian embryo is accompanied by broad, global changes in tissue architecture. Upon implantation, the primate preimplantation epiblast transits from an apolar cell mass to an epithelial flat embryonic disc. The biological consequences of the mechanical forces directing epiblast morphology on directing pluripotent states remain largely unexplored, limiting our current knowledge of appropriate substrates for use in long-term in vitro culture of primate cells. The project would investigate the effects of substrate stiffness on pluripotent status in naïve and primed marmoset ESCs.
The transition from the naïve to primed pluripotent state in the mammalian embryo is accompanied by broad, global changes in tissue architecture. Upon implantation, the primate preimplantation epiblast transits from an apolar cell mass to an epithelial flat embryonic disc. The biological consequences of the mechanical forces directing epiblast morphology on directing pluripotent states remain largely unexplored, limiting our current knowledge of appropriate substrates for use in long-term in vitro culture of primate cells. The project would investigate the effects of substrate stiffness on pluripotent status in naïve and primed marmoset ESCs.
Applications should include your cover letter, briefly outlining why you want to work with us, your CV and the names and contact details of two referees and can be sent directly to Thorsten Edwin Boroviak (teb45@cam.ac.uk).