Research focus
Life originates from a single cell, the zygote.
The zygote divides and gives rise to the embryo, a handful of loosely attached cells. Our lab focuses on how embryonic cells organise themselves to form the most complex lifeforms, such as human and non-human primates.
We follow primate embryonic cells through parts of their journey to provide insights into human development. Our approaches include simultaneous genetic and epigenetic high-throughput sequencing from single cells, embryonic stem cell culture and bioengineering of stem cell-based embryo models.
A deeper understanding of primate development is vital for innovative treatments of implantation failure, infertility and cancer as well as clinical applications of stem cell biology.
Lineage specification in the early embryo
In preimplantation development, the embryo establishes the three founding populations of the late blastocyst: pluripotent epiblast, which forms the embryo proper, and extraembryonic hypoblast and trophectoderm, which give rise to the lineages of the yolk sac and the placenta, respectively.
Trophectoderm is essential for embryo attachment and invasion into maternal tissues; the hypoblast diversifies into visceral endoderm and yolk sac.
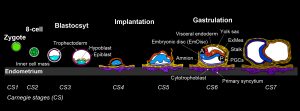
The first signs of the human body axis can be traced back to the second week of gestation. To get to this point, the blastocyst implants and establishes a small sheet of cells, the embryonic disc.
Deeply embedded within extraembryonic tissues, the embryo undergoes a reorganization process termed “gastrulation”, which transforms the embryonic disc into three germ layers and determines the entire future body plan.
Most of our knowledge on mammalian gastrulation is based on mouse1,2, but human embryogenesis differs in anatomical architecture, timing, molecular configuration and sequence of cell-fate decisions3–5.
In human and non-human primates, the implanting epiblast polarises into a rosette, gives rise to amnion and forms a flat EmDisc5,6. Rodent embryos form amnion later in development, after gastrulation.
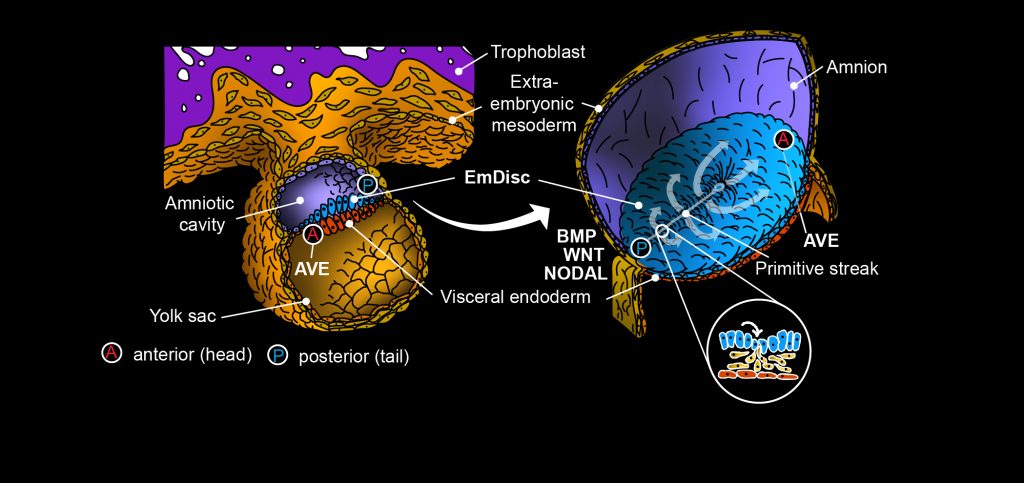
The primitive streak marks the onset of gastrulation: Cells in the posterior – the tail end – of the embryonic disc undergo epithelial-to-mesenchymal transition, ingress in between the embryonic disc and visceral endoderm, and diversify into endoderm and mesodermal lineages7–10.
Timing is critical, as within the primitive streak different domains are fated to generate distinct cell populations to manifest the body plan10–12.
Why primates are different…
Gastrulation is controlled by evolutionary conserved WNT-, BMP-, and NODAL-signalling pathways components1,12–16. In mouse, the visceral endoderm forms a dynamic signalling centre, the anterior visceral endoderm (AVE), which plays an important role for gastrulation1,2.
Soluble inhibitors from the AVE restrict gastrulation towards the opposite side of the embryo, where a combination of WNT-, BMP- and NODAL-signalling induces primitive streak formation1,2. In contrast to the established role of the rodent AVE, the function of the primate AVE in gastrulation has remained unknown.
However, embryo profiling studies indicate that pluripotent cells co-exist with gastrulating cells for over one week in the early postimplantation primate embryo12,13, in contrast to mouse17,18.
Our spatial profiling study in the marmoset embryo showed that the pluripotent population resides in the anterior embryonic disc, adjacent to the primate AVE12, and that the primate AVE expresses large amounts of NODAL (Fig. 3).
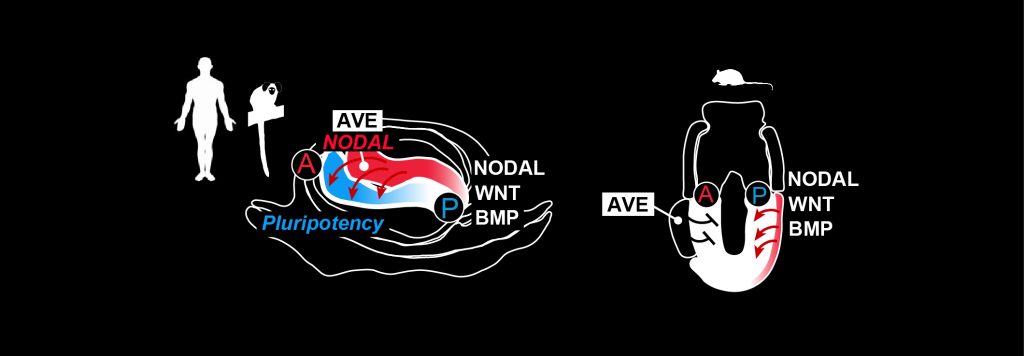
Considering the prominent role of TGFb/NODAL-signalling for self-renewal in primate PSCs12,19, it is likely that NODAL from the AVE sustains the anterior pluripotent compartment in the embryo.
Thus, we hypothesise that the primate AVE counterbalances the gastrulation-inducing forces of BMP- and WNT-signalling through NODAL, NOGGIN and CER1 expression, thereby adjusting the pace of primate gastrulation.
Equally, the primate AVE itself might be under the dynamic influence of the gastrulating embryo, possibly through WNT and NOTCH signalling pathway components.
The marmoset as a model for human development
 The common marmoset (Callithrix jacchus) is a small New World monkey and an established pre-clinical model.
The common marmoset (Callithrix jacchus) is a small New World monkey and an established pre-clinical model.
To model human development in vitro, we primarily use human pluripotent stem cells. However, including marmoset stem cells and embryo models offers an unprecedented opportunity to directly compare in vitro cultures to the implanting embryo in vivo12.
Marmoset development is relevant because it closely recapitulates human development3–5, in contrast to non-primate species.
AIMS
The differences between rodent and primate development remain poorly understood. This limits our understanding of embryo implantation, gastrulation and organogenesis in our own species.
To address these issues, we aim to…
- Delineate hypoblast and trophoblast lineage progression
- Functionally dissect embryo patterning with bioprinted gastruloids
- Emulate human and marmoset embryo implantation and postimplantation development with blastoids
- Assess the developmental potential of marmoset blastoids in vivo
The central aim of our research focuses on delineating the molecular cross-talk between the human embryonic disc and the AVE, which controls the sequence of cell-fate allocation in the primitive streak.
Recently, our lab revealed the molecular landscape in primate embryos between implantation and gastrulation in vivo (Bergmann et al., Nature 2022).
We developed epiblast- and amnion-spheroid cultures using microfluidics (Schindler et al., Stem Cell Reports 2021; Munger et al., Development 2022) and pioneered computational approaches, including spatial-identity-mapping, to determine the identity of in vitro cultured cells.
Our ongoing work involves micropatterning, blastoids, microfluidics and bioprinting to emulate human and non-human primate development in stem cell-based embryo models.
The results from our work will be critical to understand human implantation failure, how errors in gastrulation can lead to congenital malformations and how germ layers are patterned for organ formation. Ultimately, this research holds the transformative potential to establish patient-specific organogenesis in a dish.
References
- Arnold, S. J. & Robertson, E. J. Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nat Rev Mol Cell Biol 10, 91–103 (2009).
- Stower, M. J. & Srinivas, S. Heading forwards: Anterior visceral endoderm migration in patterning the mouse embryo. Philos. Trans. R. Soc. B Biol. Sci. 369, (2014).
- Rossant, J. & Tam, P. P. L. Exploring early human embryo development. Science (80-. ). 360, 1075–1076 (2018).
- Ross, C. & Boroviak, T. E. Origin and function of the yolk sac in primate embryogenesis. Nat. Commun. (2020) doi:10.1038/s41467-020-17575-w.
- Boroviak, T. & Nichols, J. Primate embryogenesis predicts the hallmarks of human naïve pluripotency. Development 144, 175–186 (2017).
- Rock, J. & Hertig, A. T. The human conceptus during the first two weeks of gestation. Am J Obs. Gynecol 55, 6–17 (1948).
- Hertig, A. T., Rock, J. & Adams, E. C. A description of 34 human ova within the first 17 days of development. Am J Anat 98, 435–493 (1956).
- Hertig, A. T. & Rock, J. On a human blastula recovered from the uterine cavity 4 days after ovulation. Anat Rec 94, 469 (1946).
- Luckett, W. P. Origin and differentiation of the yolk sac and extraembryonic mesoderm in presomite human and rhesus monkey embryos. Am. J. Anat. (1978) doi:10.1002/aja.1001520106.
- Tyser, R. C. V. et al. Single-cell transcriptomic characterization of a gastrulating human embryo. Nature 600, (2021).
- Lawson, K. A., Meneses, J. J. & Pedersen, R. A. Clonal analysis of epiblast fate during germ layer formation in the mouse embryo. Development 113, 891–911 (1991).
- Bergmann, S. et al. Spatial profiling of early primate gastrulation in utero. Nature (2022).
- Nakamura, T. et al. A developmental coordinate of pluripotency among mice, monkeys and humans. Nature 537, 57–62 (2016).
- Sasaki, K. et al. The Germ Cell Fate of Cynomolgus Monkeys Is Specified in the Nascent Amnion. Dev Cell (2016) doi:10.1016/j.devcel.2016.09.007.
- Takaoka, K. & Hamada, H. Cell fate decisions and axis determination in the early mouse embryo. Development (2011) doi:10.1242/dev.060095.
- Hassoun, R., Schwartz, P., Feistel, K., Blum, M. & Viebahn, C. Axial differentiation and early gastrulation stages of the pig embryo. Differentiation (2009) doi:10.1016/j.diff.2009.07.006.
- Peng, G. et al. Molecular architecture of lineage allocation and tissue organization in early mouse embryo. Nature (2019) doi:10.1038/s41586-019-1469-8.
- Lawson, K. A. Fate mapping the mouse embryo. Int J Dev Biol 43, 773–775 (1999).
- Vallier, L. et al. Activin/Nodal signalling maintains pluripotency by controlling Nanog expression. Development (2009) doi:10.1242/dev.033951.
- Kinder, S. J. et al. The orderly allocation of mesodermal cells to the extraembryonic structures and the anteroposterior axis during gastrulation of the mouse embryo. Development (1999).
- Bianchi, D. W., Wilkins-Haug, L. E., Enders, A. C. & Hay, E. D. Origin of extraembryonic mesoderm in experimental animals: Relevance to chorionic mosaicism in humans. Am. J. Med. Genet. (1993) doi:10.1002/ajmg.1320460517.
- Enders, A. C. & King, B. F. Formation and differentiation of extraembryonic mesoderm in the rhesus monkey. Am J Anat 181, 327–340 (1988).